Highly Efficient Chemoselective Deprotection of O, O-Acetals and O, O-Ketals Catalyzed by Molecular Iodine in Acetone | PDF | Iodine | Ketone

PDF) A Simple and Versatile Method for the Synthesis of Acetals from Aldehydes and Ketones Using Bismuth Triflate | Ram Mohan - Academia.edu
PDF) Stereoarrayed CF 3 -Substituted 1,3-Diols by Dynamic Kinetic Resolution: Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier

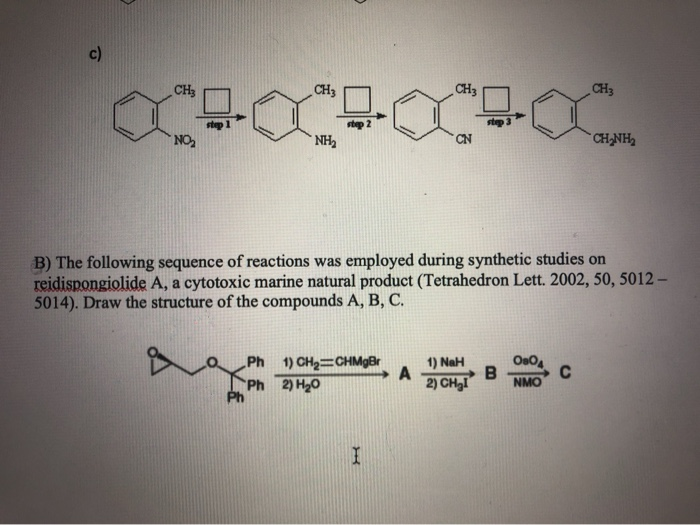
SOLVED: The following sequence of reactions was employed during synthetic studies on reidispongiolide A, a cytotoxic marine natural product (Tetrahedron Lett. 2009, 50, 5012-5014). Draw the structures of compounds A, B, C,

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier

SOLVED: Draw the final products for the following reactions shown below and explain your answers to get full credits. CHO TFA . TF1,0 Am Chem Soc 2001 123,4370 POCl3; DMF Tetrahedron Lett.
PPT - Kwart, H.; Khan, A. A. J. Am. Chem. Soc. 1967, 89 , 1951–1953. Breslow, R.; Gellman, S. H. J. Chem. Soc., Chem. Com PowerPoint Presentation - ID:796195

Tetrahedron Letters | Vol 43, Issue 50, Pages 8993-9273 (9 December 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 35, Pages 6083-6279 (26 August 2002) | ScienceDirect.com by Elsevier

PDF) The Baylis-Hillman reaction: One-pot facile synthesis of 2, 4-functionalized 1, 4-pentadienes | Kumaragurubaran Nagaswamy - Academia.edu

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier